Redefine how you create, manage and print labels
Loftware helps businesses of all sizes manage labeling across their operations and supply chain. Whether you have five printers or thousands, looking for cloud or on-premise, we've got a labeling solution that fits your business requirements.

The industry’s most comprehensive labeling solutions
All-in-one labeling solutions
By standardizing the entire label printing process into one platform, Loftware redefines how organizations manage complex and mission-critical labeling operations.
Certified integrations with business systems
With Loftware’s certified integrations to leading business applications, such as Oracle and SAP, you can connect labeling with any of your existing business systems to streamline and automate your labeling processes.
Labeling built for the Cloud
Loftware’s labeling solutions are designed to be run in the cloud, helping you achieve the highest level of print performance and reliability for your mission critical labeling.
Putting label management in the hands of business users
Our intuitive interfaces and WYSIWYG design capabilities make it easy for business users to manage the label design process. This frees up costly IT resources and creates a more efficient label design and management process.
Meeting your unique labeling requirements
You can configure our labeling solutions to support your business processes and to satisfy specific customer, industry and regulatory requirements. Our solutions also include pre-designed label templates and applications that comply with common industry regulations.
Industry best practices and first-class service
With extensive services, support and training available for all your labeling needs, we are always here when you need us…anytime, anywhere - around the globe.
Loftware fundamentally transforms legacy approaches to labeling
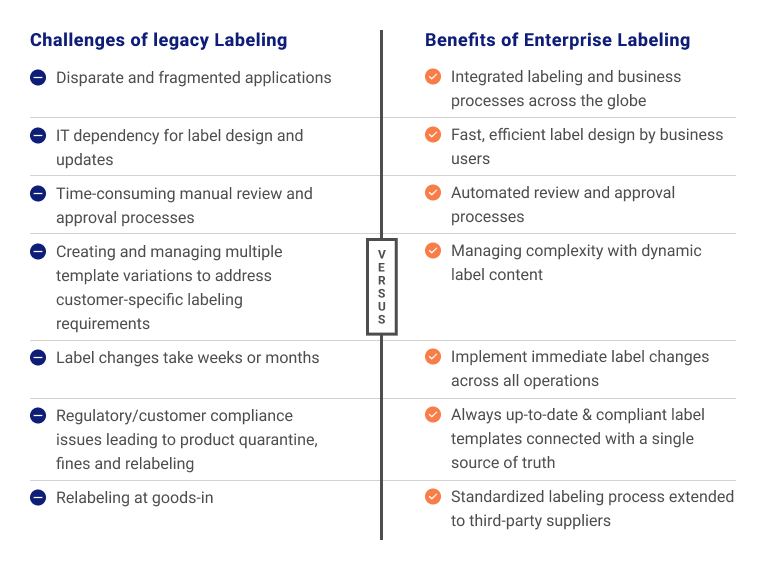
The right solution for your business
As leaders in labeling, we create innovative solutions that address the full range of barcode labeling and artwork management requirements for companies of all sizes and across a broad range of industries.

For single site, smaller business
Quickly produce professional labels that meet the most common business requirements. Designing labels has never been so easy.

For mid-size and growing businesses
From label design to print – get everything your growing business needs to easily manage labeling. Designed for rapid deployments at one site or across multiple locations.

For enterprises
Centralize and standardize your global labeling with an all-in-one labeling solution. Seamlessly integrate, manage and scale your labeling across global operations and supply chains.

For clinical trials
Meet the strictest compliance requirements with the industry’s most powerful clinical label and booklet management solution. Built for clinical trial organizations.

For medical device small and mid-sized businesses
Easily manage labeling in a regulated environment with a validation-ready cloud labeling solution.

For medical device enterprises
Create and maintain a compliant labeling process with a solution, specifically designed to help medical device manufacturers meet rigorous regulatory requirements.
Recommended Resources
Case Study
Loftware helps Autoliv standardize labeling and cut label design time in half
Loftware helps Autoliv standardize labeling and cut label design time in half. The main benefit Autoliv has experienced...
Report
7 Business-Boosting Benefits of Labeling in the Cloud
The cloud has hands-down become the simplest way for companies of all sizes to design, manage and print labels across...
Webinar
How Zumtobel Leveraged the Cloud for a More Reliable Labeling Process
Watch this recorded webinar where we spoke to Thomas Muller, Head of IT Solutions Manufacturing & Logistics at Zumtobel...
White Paper
Fashion's sustainable evolution: Navigating regulatory compliance with the Cloud
Download this whitepaper to learn how cloud-based labeling helps fashion retailers to ensure supply chain traceability,...